Categories
- Global News Feed
- Uncategorized
- Alabama Stem Cells
- Alaska Stem Cells
- Arkansas Stem Cells
- Arizona Stem Cells
- California Stem Cells
- Colorado Stem Cells
- Connecticut Stem Cells
- Delaware Stem Cells
- Florida Stem Cells
- Georgia Stem Cells
- Hawaii Stem Cells
- Idaho Stem Cells
- Illinois Stem Cells
- Indiana Stem Cells
- Iowa Stem Cells
- Kansas Stem Cells
- Kentucky Stem Cells
- Louisiana Stem Cells
- Maine Stem Cells
- Maryland Stem Cells
- Massachusetts Stem Cells
- Michigan Stem Cells
- Minnesota Stem Cells
- Mississippi Stem Cells
- Missouri Stem Cells
- Montana Stem Cells
- Nebraska Stem Cells
- New Hampshire Stem Cells
- New Jersey Stem Cells
- New Mexico Stem Cells
- New York Stem Cells
- Nevada Stem Cells
- North Carolina Stem Cells
- North Dakota Stem Cells
- Oklahoma Stem Cells
- Ohio Stem Cells
- Oregon Stem Cells
- Pennsylvania Stem Cells
- Rhode Island Stem Cells
- South Carolina Stem Cells
- South Dakota Stem Cells
- Tennessee Stem Cells
- Texas Stem Cells
- Utah Stem Cells
- Vermont Stem Cells
- Virginia Stem Cells
- Washington Stem Cells
- West Virginia Stem Cells
- Wisconsin Stem Cells
- Wyoming Stem Cells
- Biotechnology
- Cell Medicine
- Cell Therapy
- Diabetes
- Epigenetics
- Gene therapy
- Genetics
- Genetic Engineering
- Genetic medicine
- HCG Diet
- Hormone Replacement Therapy
- Human Genetics
- Integrative Medicine
- Molecular Genetics
- Molecular Medicine
- Nano medicine
- Preventative Medicine
- Regenerative Medicine
- Stem Cells
- Stell Cell Genetics
- Stem Cell Research
- Stem Cell Treatments
- Stem Cell Therapy
- Stem Cell Videos
- Testosterone Replacement Therapy
- Testosterone Shots
- Transhumanism
- Transhumanist
Archives
Recommended Sites
Category Archives: Biotechnology
The Numeric Investors LLC Purchases New Position in Puma Biotechnology Inc (PBYI) – Petro Global News 24
Posted: March 15, 2017 at 5:48 am
Numeric Investors LLC acquired a new stake in Puma Biotechnology Inc (NYSE:PBYI) during the fourth quarter, Holdings Channel reports. The fund acquired 37,200 shares of the biopharmaceutical companys stock, valued at approximately $1,142,000.
A number of other hedge funds and other institutional investors also recently bought and sold shares of PBYI. Tower Research Capital LLC TRC boosted its stake in shares of Puma Biotechnology by 253.3% in the third quarter. Tower Research Capital LLC TRC now owns 2,427 shares of the biopharmaceutical companys stock valued at $163,000 after buying an additional 1,740 shares during the last quarter. Legal & General Group Plc boosted its stake in shares of Puma Biotechnology by 142.4% in the second quarter. Legal & General Group Plc now owns 6,142 shares of the biopharmaceutical companys stock valued at $183,000 after buying an additional 3,608 shares during the last quarter. Ardsley Advisory Partners acquired a new stake in shares of Puma Biotechnology during the third quarter valued at approximately $201,000. Laurion Capital Management LP acquired a new stake in shares of Puma Biotechnology during the third quarter valued at approximately $208,000. Finally, Perceptive Advisors LLC acquired a new stake in shares of Puma Biotechnology during the fourth quarter valued at approximately $225,000. Hedge funds and other institutional investors own 80.98% of the companys stock.
Shares of Puma Biotechnology Inc (NYSE:PBYI) traded up 3.37% during trading on Monday, hitting $41.40. 210,498 shares of the stock traded hands. The firms market capitalization is $1.53 billion. Puma Biotechnology Inc has a 1-year low of $19.74 and a 1-year high of $73.27. The firm has a 50-day moving average of $35.26 and a 200-day moving average of $44.21.
Puma Biotechnology (NYSE:PBYI) last issued its earnings results on Wednesday, March 1st. The biopharmaceutical company reported ($2.04) earnings per share for the quarter, missing analysts consensus estimates of ($1.92) by $0.12. On average, equities analysts forecast that Puma Biotechnology Inc will post ($8.32) earnings per share for the current year.
PBYI has been the subject of several recent research reports. Citigroup Inc set a $88.00 target price on shares of Puma Biotechnology and gave the company a buy rating in a research report on Tuesday, January 3rd. JPMorgan Chase & Co. set a $89.00 target price on shares of Puma Biotechnology and gave the company a buy rating in a research report on Monday, November 14th. Royal Bank of Canada reiterated a sector perform rating and set a $17.00 target price (down previously from $48.00) on shares of Puma Biotechnology in a research report on Thursday, March 2nd. Credit Suisse Group AG reiterated an outperform rating and set a $111.00 target price on shares of Puma Biotechnology in a research report on Tuesday, November 15th. Finally, Stifel Nicolaus reiterated a buy rating and set a $88.00 target price on shares of Puma Biotechnology in a research report on Wednesday, November 30th. One equities research analyst has rated the stock with a sell rating, three have issued a hold rating and four have given a buy rating to the company. The company presently has an average rating of Hold and a consensus price target of $73.50.
In other Puma Biotechnology news, insider Alan H. Auerbach sold 10,202 shares of the firms stock in a transaction that occurred on Friday, January 20th. The shares were sold at an average price of $33.24, for a total value of $339,114.48. Following the sale, the insider now owns 4,179,798 shares in the company, valued at $138,936,485.52. The sale was disclosed in a document filed with the Securities & Exchange Commission, which is accessible through this link. Also, SVP Richard Paul Bryce sold 2,293 shares of the firms stock in a transaction that occurred on Friday, January 20th. The shares were sold at an average price of $33.24, for a total transaction of $76,219.32. Following the completion of the sale, the senior vice president now owns 29,237 shares in the company, valued at approximately $971,837.88. The disclosure for this sale can be found here. Insiders sold 15,503 shares of company stock worth $511,078 in the last 90 days. Company insiders own 22.70% of the companys stock.
Puma Biotechnology Company Profile
Puma Biotechnology, Inc is a biopharmaceutical company that focuses on the development and commercialization of products for the treatment of cancer. The Company focuses on in-licensing the global development and commercialization rights to over three drug candidates, including PB272 (neratinib (oral)), which the Company is developing for the treatment of patients with human epidermal growth factor receptor type 2 (HER2), positive breast cancer, and patients with non-small cell lung cancer, breast cancer and other solid tumors that have a HER2 mutation; PB272 (neratinib (intravenous)), which the Company is developing for the treatment of patients with advanced cancer, and PB357, which is an orally administered agent.
Want to see what other hedge funds are holding PBYI? Visit HoldingsChannel.com to get the latest 13F filings and insider trades for Puma Biotechnology Inc (NYSE:PBYI).
Posted in Biotechnology
Comments Off on The Numeric Investors LLC Purchases New Position in Puma Biotechnology Inc (PBYI) – Petro Global News 24
Why Should Investors Consider An Allocation In Speculative Biotechnology: A Sector Analysis – Seeking Alpha
Posted: March 14, 2017 at 2:48 pm
Many intriguing articles have been written about investing in biotechnology. Biotechnology investment has been referenced by many knowledgeable and respectable authors as controversial, out of favor, and even sexy (this article by Stephen Simpson, CFA, is must read). Surviving current trends in biotechnology stock price manipulation can be both stressful and disappointing. This leaves us all to wonder is it even worth it to try speculative biotechnology as an investment option?
StrongBio believes it is worth it, even if it results in losses that are hard to endure. In the end, contributions to healthcare from growing sources of capital are extremely important for improved patient care (termed supply-side capital). As these contributions have grown, however, so too has waste. With proper selection, timing, and diversification (three pillars of biotechnology investment), the common retail investor can eventually be financially rewarded as philanthropic goals of the population are met.
Simply put, biotechnology companies focus on drug development aiming to treat an unmet or under-met disease or medical condition. Companies that have succeeded have net sales in the tens of billions and total market values in the hundreds of billions of dollars. Speculative biotechnology companies, in contrast, differ from proven top biotechnology companies in that they often have no approved products or revenues.
Gary Pisano of Harvard Business School has done extensive modeling of biotechnology (and other technology) returns. Reports between 2006 and 2012 indicate that average biotechnology returns have been historically unimpressive; with 25-year returns of "market baskets" of biotech stocks yielding only about 10% per year. This means that much of the legendary "opportunity" in biotechnology stocks revolves around successful portfolio management of technological trend shifts and timing positions accordingly.
Much of the challenges lie in the fact that science experts tend to focus into niches instead of pursue interdisciplinary science. Scientists tend to lack fundamental economics or business expertise and vice-versa, with business leaders lacking science background.
So what if a speculative biotechnology company has shown positive data in a curative treatment for cancer? Many things can still go wrong for an investor. One should always have a plan for setbacks and delays. Sometimes clinical setbacks can occur requiring a company to delay a trial until regulatory requirements are met. Other delays are more business-oriented, with slated clinical trials held up due to lack of funding such as in poor economic times.
Even legal setbacks occur and can cost both time and money. And then we have the gatekeeper: FDA, and regulatory setbacks that can occur. The fact is most biotechnology projects fail. According to Pisano, the average biotechnology company is likely to fail 90% of the time, with companies that make it all the way to Phase 3 experiencing approximately 50% chance of success.
Multidisciplinary investment management increases the likelihood of a success, meaning, that many common retail investors are going to have to try to wear multiple hats when performing qualitative analyses. That's what StrongBio calls work. It's a lot of work. But knowing what to look for in each discipline can be of great service to the retail investor.
And if all of those pitfalls are not enough, going back to our legitimate cancer data success scenario, market manipulation and fake news from negative press can still make investors feel like their winners are losing investments for quite some time. Take for instance the 2016 situation with Celator Pharmaceuticals, which was driven down to $0.79 cents per share and rose 1600% when whatever market forces that were holding it down, along with negative press, finally gave up the fight to an obvious winner (having been bought out by Jazz Pharmaceuticals (NASDAQ:JAZZ).
The oppressive forces on the stock persisted right up until FDA review. Other company shareholders, like those of Northwest Biotherapeutics (NASDAQ:NWBO), allege that negative press and stock manipulation are linked. Immunomedics's (NASDAQ:IMMU) stock see-sawed back and forth several times between $2 and $5 (and even drew a halt from the SEC), market cap between 180 million and 500 million respectfully, in 2016 with alternating negative legal press by no less than 20 law firms and positive research press and stock price volatility. Extreme patience is required while waiting for "fair value."
The University of Chicago oncologist Mark Ratain postulated that a company with a market cap of less than $300 million is unlikely to succeed. Commonly known in biotechnology investment circles, the Feuerstein-Ratain rule, was a solid predictor in the past. This year companies are defying the 300 million rule. StrongBio believes the rule used to hold water because there was a predictable method to the involvement of big pharma in purchasing speculative biotechnology cancer stocks.
So either big pharma is no longer able to identify useful technologies and many are slipping through the cracks, which is unlikely, or something in the markets is changing. It is also possible that it was getting predictable to pick biotechnology successes based upon the highly successful metric by the well-respected Feuerstein and Ratain, so market makers have changed it up a little. Past open market buyout periods of obtaining shares of speculative stocks drove prices up as a whole (or held them flat for long periods of time) to approximately 300 million market caps. Accumulation such as this no longer seems to be in effect.
Whatever the mechanism of value assignment by the market, it is clear there is a new market pattern emerging in biotechnology, with lower than normal market caps. StrongBio believes there may be several contributing reasons for this. One, investment levels are predicted to be the lowest that they have been since 1947. This is also true in the investment banking sector, a big source of biotechnology funding.
Simply put we have not had a great investment economy, and risky biotechnology may be regarded as an irresponsible investment during tough financial times. Because new patterns on speculative biotechnology company stocks show suppression over periods of months and years, it is possible there just is not as much retail and/or institutional support as in years past.
Two, is the SEC unconscious at the wheel or did someone outsource that job to Asia? This query addresses concern over foreign-based or hostile entities' ability to starve funding for cash hungry technology companies when they need to sell stock. In recent years, an increase in foreign companies cheaply acquiring U.S. biotechnologies developed at tax-payer-supported universities and other technologies funded by state and local governments has plagued markets.
New stock exchanges like IEX have even been set up in an attempt to thwart different kinds of financial manipulation utilizing delays in trade execution (read this book or a synopsis about it in Flash Boys; it's fascinating). However, the market is currently responding to new executive political leadership in a corrective way. One can always hope that a nation of laws will have proper enforcement.
Three, short sellers have influenced stock prices (being respectful of regulations) for a long time, but not to the extent that manipulation is occurring now. Foreign countries like those in Western Europe, Australia and Canada have entirely outlawed the practice of shorting on their own stock exchanges. This indicates these countries have identified that stocks were oversold and manipulated and regulations and laws limiting short sales were not able to control it.
It would appear pretty obvious to those following speculative biotechnology that the same is occurring in the U.S. For instance, one exploring and mining company presented evidence to the SEC of a naked short position in the hundreds of billions of shares. Regardless of mechanism there may be a way to estimate increases in short-selling using well established metrics in biotechnology.
StrongBio cites the 2016 failures (and likely more in 2017) of the long-established Feuerstein-Ratain rule as evidence NOT that the metric is somehow flawed, but rather that market conditions have changed. The 300 million "rule" is now as dated as 4-inch tile countertops for kitchens and bathrooms, and has likely been rendered obsolete by rampant stock price manipulation. But that does not mean that one should abandon biotechnology investments.
Eventually, a fair value is decided between a suitor and company management if something in the pipeline passes FDA and can be sold. Since Celator (NASDAQ:CPXX) rendered the Feuerstein-Ratain rule obsolete at a market cap of less than $100 million, and Immunomedics obtained FDA breakthrough therapy status in triple negative breast cancer at about $160 million, we know the static threshold value of 300 million is no longer even close to critical mass for the metric.
Out of fairness, the metric can be influenced by other factors such as a changing FDA landscape as well, but that wouldn't explain the difference in market cap as the FDA does not participate in stock pricing or market making per se. It follows that if market regulation returns to prior levels, the metric threshold would increase back to 300 million.
How much lower can the Feuerstein-Ratain critical threshold go? That may depend in part how many shares can be shorted in a given company. StrongBio cites here mention of a gnarly cancer drug company from a recent article by an author of the metric that has approvable Phase 3 data. According to a report by H.C. Wainright, this 40 million market cap company, CytRx (NASDAQ:CYTR), is likely to get some kind of approval pathway for aldoxorubicin on its statistically significant Phase 3 sarcoma data, where it outperformed all 5 other drugs in the study.
One might approximate that if short selling is the cause for lower market cap FDA approvals in cancer, estimates based upon how much additional share supply exists now versus when the rule was working can be made. 300 million, the past metric threshold, divided by 40 million, the current potential low, gives a WHOPPING 7.5-fold more potential share supply (provided to the market by short selling). This assumes that the relationship between stock price and shorting of shares has some kind of linear and direct relationship, such as common economic supply and demand curves.
This implies the Feuerstein-Ratain metric tool is a dynamic sliding-threshold subject to changing market factors. One might argue that a linear relationship would be less representative for a "real world" sigmoidal supply and demand model, such as that proposed by Alfred Marshall. These curves break from a linear path to form a smooth parabolic curve with sigmoid limits because of factors such as wear and tear of production equipment, transportation limits, and other practical factors in supply-side analysis. In an electronic market system of a thinly traded biotechnology stock, it is unlikely these factors are relevant.
This emerging scenario creates an even greater margin for profit if one can properly select stocks that are at a record low market cap threshold for FDA approval in cancer, sometimes called short squeeze. At some point, speculative biotechnology companies get a fair value assigned if management is honest enough to serve their fiduciary duty to shareholders. It is at the point of buyout that fair value was reached for CPXX. Fair value soon will be reached for IMMU based upon significant partnership (Seattle Genetics, SGEN) and possibly even CYTR.
StrongBio cautions the reader that CytRx has been accused of hiring stock promotion media in the past, and its pillar of honor might have been "pierced." Nonetheless it appears that after a substantial period of risk everything that was promoted is likely to be true. In addition, remember that biotechnology investment in a "market basket" of companies typically returns about 10%. StrongBio does not recommend deviating from this basket approach, but rather by changing weighting late into development and after dilutions, obtain higher than 10% returns when possible.
The primary pillar of biotechnology stock investing, selection, is obviously a critical factor, standing tall in front of the pillar of timing. How can we avoid picking a loser? The second pillar of timing comes down to choosing a company with favorable Phase 3 data as they meet with FDA in a type B meeting (where FDA reviews the data outcomes of a clinical study) and an abnormally low stock price compared to the annual market its product will serve.
These two pillars stand in synergism, as one doesn't have a favorable investment with only one solid pillar supporting a portfolio candidate under current market conditions. It is important to invest lightly at first so that lower prices do not cause harm to a portfolio. If the stock runs up just be happy you had a little.
The pitfall of regulatory hurdles is always a major concern, but there is circumstantial evidence that the FDA will be easing some burdens. Cancer drug shortages (such as existed for doxorubicin in December 2016) can be thwarted by increasing the number of suppliers and approving safer more effective derivatives. The FDA may favor competition to lower prices of potentially egregious monopolies. Cancer treatment in the hospital environment is currently trending towards increasing physician options and information as well as for patient-physician interaction as well.
For instance, some drugs may be hard on the liver and not be good for alcoholics, whereas other drugs may be rough on the heart, and be contra-indicated for heart attack sufferers. So demonstration of comparable efficacy may be acceptable if safety is improved for subsets of patients. Whether the desire for increased options will spread from cancer to other indications remains to be seen.
However it is no secret that Trump intends to "slash restraints" artificially put on drug makers by the FDA. If these trends come to fruition, StrongBio expects the chance of success of biotechnology companies to increase, but the markets for some drugs may sink into smaller niches of sales with greater total options available.
So there are certainly the same past investment risks that the FDA will not view data as favorable or that companies will have a hard time proving they can meet production standards for NDA approval, including lot to lot consistency. Oftentimes a speculative biotechnology company can partner this production with a number of firms.
But the reward to risk ratio can at least be dynamically tuned for investment success. With proper selection, timing, and diversification, StrongBio estimates that new regulatory policies and market conditions will make biotechnology investment potentially more common and successful as the outdated thresholds of the past are readjusted to guide investors.
Disclosure: I am/we are long IMMU CYTR.
I wrote this article myself, and it expresses my own opinions. I am not receiving compensation for it (other than from Seeking Alpha). I have no business relationship with any company whose stock is mentioned in this article.
Editor's Note: This article covers one or more stocks trading at less than $1 per share and/or with less than a $100 million market cap. Please be aware of the risks associated with these stocks.
Go here to see the original:
Why Should Investors Consider An Allocation In Speculative Biotechnology: A Sector Analysis - Seeking Alpha
Posted in Biotechnology
Comments Off on Why Should Investors Consider An Allocation In Speculative Biotechnology: A Sector Analysis – Seeking Alpha
Have Biotech ETFs Finally Bottomed? – ETF Daily News (blog)
Posted: March 14, 2017 at 2:48 pm
March 14, 2017 7:54am NASDAQ:IBB
From Zacks: 2016 was a tough year for biotech stocks with the sector facing a lot of criticism for rising drug prices. Although shares did rally post-election in November on hopes that drug pricing would not be a key focus area under a Donald Trump presidency, the rally turned out to be short-lived following the Presidents views regarding drug pricing.
Trump made it clear that he does not like what happened to drug prices and he will bring them down.
Drug pricing was not the only issue that impacted the sector last year 2016 was also disappointing from an R&D perspective with a fewer number drugs managing to gain FDA approval. There were some high-profile pipeline failures as well. Other factors that weighed on the sector include mixed results, slower-than-expected new product launches and increasing competition.
The impact of these issues resulted in the NASDAQ Biotechnology Index declining 19.1% in 2016. However, the sector is showing signs of recovery this year with the index gaining 12.4%year-to-date (YTD). (Read: Top-Ranked ETFs That Crushed S&P 500 in the Bull Market)
Drug Pricing Will Remain a Headline Risk
With drug pricing being a populist issue, it looks like the spotlight will remain on rising drug prices in 2017 as well. According to the Jan 2017 Kaiser Health Tracking poll, affordability of prescription drugs remains at the top of the publics priority list for the President and Congress focus should be on ensuring the affordability of high-cost drugs to people who need them and taking steps to lower prescription drug prices.
President Trumps recent tweet that he is working on a new system where there will be competition in the Drug Industry and pricing for the American people will come way down will keep the biotech industry on edge. While it is clear that the government intends to address the drug pricing issue, there is no clarity on what steps will be taken.
Biosimilars Pose a New Threat
Another challenge being faced by the sector is the recent entry of biosimilar competition in the U.S. While a relatively new area, the market for biosimilars is huge and highly lucrative with several blockbuster biologics including Humira and Lantus slated to lose patent protection by 2020. Biosimilars are expected to reduce healthcare costs and provide a large number of patients with access to much needed biologic treatments. Biosimilars are also gaining acceptance across formularies. (Read: Trumps Defense Spending Plans Make These ETFs Buys Again)
Deals to Pick Pace?
Licensing agreements and deals including those with opt-in arrangements should continue being signed with immuno-oncology remaining a favorite area. Moreover, major biotech and pharma companies should gain from Trumps proposed tax plan and proposal to repatriate corporate profits held offshore at a one-time tax rate.
Given the possibility of repatriation of funds, chances are that M&A activity will pick up as the year progresses big companies with deep pockets often look to replenish and boost their pipelines as well as portfolios by acquiring companies with innovative pipelines and technology. Meanwhile, small bolt-on acquisitions will continue. (Read: Play These Stocks & ETFs If Fed Acts in March)
Companies with innovative technologies and pipelines are highly sought after. Niche disease areas like nonalcoholic steatohepatitis (NASH), immuno-oncology and multiple sclerosis are in demand. Treatments for orphan diseases are also much sought after with quite a few deals being signed in these areas.
New Products Should Gain Traction
Highly-awaited new products that gained approval over the last couple of years should contribute significantly to revenues. The FDA approved 22 new drugs in 2016 including Exondys 51 (Duchenne muscular dystrophy), Epclusa (hepatitis C virus), Ocaliva (rare, chronic liver disease), Zinbryta (multiple sclerosis), and Venclexta (chronic lymphocytic leukemia in patients with a specific chromosomal abnormality) among others. The agency also expanded the label of cancer drugs like Kyprolis and Imbruvica.
Meanwhile, so far in 2017, the FDA has approved 5 new drugs including Trulance (treatment of chronic idiopathic constipation in adults) and Parsabiv (treatment of secondary hyperparathyroidism in adult patients with chronic kidney disease undergoing dialysis).
ETFs in Focus
Highlighted below are some biotech ETFs ETFs present a low-cost and convenient way to get a diversified exposure to the sector.
iShares Nasdaq Biotechnology (IBB)
IBB, launched in Feb 2001 by BlackRock Investments LLC, tracks the Nasdaq Biotechnology Index. The fund mainly covers biotech stocks (81.6%) with pharma accounting for 10.8%, life sciences tools & services for 7.3%, Health care technology for 0.1%, Health care equipment for 0.11% and Health care supplies for 0.07%. The top 3 holdings include Amgen Inc. (9.23%), Celgene Corporation (7.72%) and Biogen Inc. (7.42%). The total assets of the fund as of Mar 7, 2017 were $8.38 billion representing 162 holdings. The funds expense ratio is 0.47% while dividend yield is 0.16%. The trading volume is roughly 1,491,728 shares per day.
SPDR S&P Biotech ETF (XBI)
XBI, launched in Jan 2006 by State Street Global Advisors, tracks the S&P Biotechnology Select Industry Index. The fund covers health care stocks only. The top 3 holdings include ARIAD Pharmaceuticals, Inc. (3.98%), Clovis Oncology, Inc. (3.76%), and ACADIA Pharmaceuticals Inc. (2.74%). The total assets of the fund as of Mar 8, 2017 were $2.9 billion representing 88 holdings. The funds expense ratio is 0.35% while dividend yield is 0.21%. The trading volume is roughly 4,427,722 shares per day.
First Trust NYSE Arca Biotech ETF (FBT)
FBT, launched in Jun 2006 by First Trust Advisors, tracks the NYSE Arca Biotechnology Index. The top 3 holdings include Kite Pharma, Inc. (4.77%), Alnylam Pharmaceuticals, Inc. (3.98%) and Nektar Therapeutics (3.77%). The total assets of the fund as of Mar 7, 2017 were $902 million representing 30 holdings. The funds expense ratio is 0.55% while dividend yield is nil. The trading volume is roughly 44,417 shares per day.
VanEck Vectors Biotech ETF (BBH)
BBH, launched in Dec 2011 by Van Eck, tracks the Market Vectors US Listed Biotech 25 Index. The fund covers health care stocks. The top 3 holdings include Amgen Inc. (12.13%), Celgene Corporation (10.73%) and Gilead Sciences Inc. (10.72%). The total assets of the fund as of Mar 8, 2017 were $702.5 million representing 26 holdings. The funds expense ratio is 0.35% while dividend yield is 0.21%. The trading volume is roughly 50,198 shares per day.
PowerShares Dynamic Biotech & Genome ETF (PBE)
PBE, launched in Jun 2005 by Invesco PowerShares, tracks the Dynamic Biotech & Genome Intellidex Index. The top 3 holdings include Incyte Corporation (5.38%), Vertex Pharmaceuticals Inc. (5.19%), and Regeneron Pharmaceuticals, Inc. (5.1%). The total assets of the fund as of Mar 8, 2017 were $245.6 million representing 31 holdings. The funds expense ratio is 0.50% while dividend yield is 0.34%. The trading volume is roughly 27,517 shares per day.
Conclusion
Although it may take a while for the dust around the drug pricing issue to settle down, pipeline success in innovative and important therapeutic areas, cost-cutting, share buybacks, new products, increased pipeline visibility and appropriate utilization of cash should help restore investor confidence in biotech stocks.
The iShares NASDAQ Biotechnology Index ETF (NASDAQ:IBB) fell $0.66 (-0.22%) in premarket trading Tuesday. Year-to-date, IBB has gained 13.28%, versus a 6.39% rise in the benchmark S&P 500 index during the same period.
IBB currently has an ETF Daily News SMART Grade of A (Strong Buy), and is ranked #2 of 36 ETFs in the Health & Biotech ETFs category.
This article is brought to you courtesy of Zacks Research.
Tags: biotech Equity Health Care NASDAQ:IBB Zacks
Categories: NASDAQ:IBB
Excerpt from:
Have Biotech ETFs Finally Bottomed? - ETF Daily News (blog)
Posted in Biotechnology
Comments Off on Have Biotech ETFs Finally Bottomed? – ETF Daily News (blog)
National Academies Report Finds Future Biotechnology Products May Overwhelm Agencies – The National Law Review
Posted: March 14, 2017 at 2:48 pm
On March 9, 2017, the National Academies of Sciences, Engineering, and Medicine (National Academies) published a report entitled Preparing for Future Products of Biotechnology, prepared by the Committee on Future Biotechnology Products and Opportunities to Enhance Capabilities of the Biotechnology Regulatory System (Committee). The Committee was asked to describe the possible future products of biotechnology that will arise over the next five to ten years, as well as provide some insights that can help shape the capabilities within the U.S. Environmental Protection Agency (EPA), the U.S. Food and Drug Administration (FDA), and the U.S. Department of Agriculture (USDA) as they move forward. According to the Committee, agencies may be overwhelmed by the number and diversity of new biotechnology products. The Committee states that the agencies should increase their scientific capabilities, tools, and expertise in key areas of expected growth. The report reflects the Committees deliberations regarding the future products of biotechnology that are likely to appear on the horizon, the challenges that the regulatory agencies might face, and the opportunities for enhancing the regulatory system to prepare for what might be coming. The Committee reached consensus on conclusions and recommendations that are based on extensive information gathering, Committee discussions, and input from a wide variety of communities interested in biotechnology. A copy of the slides used during a National Academies webinar on the report can be found on the National Academies website.
On July 2, 2015, the White House Office of Science and Technology Policy, the Office of Management and Budget, the U.S. Trade Representative, and the Council on Environmental Quality issued a memorandum, Modernizing the Regulatory System for Biotechnology Products, directing EPA, FDA, and USDA to update the Coordinated Framework for the Regulation of Biotechnology (Coordinated Framework). The Obama Administration asked the agencies to accomplish three tasks:
Clarify the current roles and responsibilities of the EPA, FDA, and USDA in the regulatory process;
Develop a long-term strategy to ensure that the federal regulatory system is equipped to assess efficiently the risks, if any, of the future products of biotechnology; and
Commission an expert analysis of the future landscape of biotechnology products.
As reported previously, on January 4, 2017, the White House announced the release of the 2017 Update to the Coordinated Framework for the Regulation of Biotechnology. The 2017 Update provides a comprehensive summary of the roles and responsibilities of EPA, FDA, and USDA with respect to regulating biotechnology products. Together with the National Strategy for Modernizing the Regulatory System for Biotechnology Products, published in September 2016, the 2017 Update offers a complete picture of a robust and flexible regulatory structure that provides appropriate oversight for all products of modern biotechnology. Within that regulatory structure, the federal agencies maintain high standards that, based on the best available science, protect health and the environment, while also establishing transparent, coordinated, predictable and efficient regulatory practices. More information is available in the White House blog item, Increasing the Transparency, Coordination, and Predictability of the Biotechnology Regulatory System.
The July 2, 2015, memorandum called for the commission of an external, independent analysis of the future landscape of biotechnology products. EPA, FDA, and USDA commissioned the National Academies to prepare an analysis to identify potential new risks and frameworks for risk assessment and areas in which the risks or lack of risks relating to the products of biotechnology are well understood. This analysis is presented in the report prepared by the Committee that was released on March 9, 2017.
The Committee was tasked to:
Describe the major advances and the potential new types of biotechnology products likely to emerge over the next five to ten years;
Describe the existing risk-analysis system for biotechnology products including, but perhaps not limited to, risk analyses developed and used by EPA, USDA, and FDA, and describe each agencys authorities as they pertain to the products of biotechnology;
Determine whether potential future products could pose different types of risks relative to existing products and organisms. Where appropriate, identify areas in which the risks or lack of risks relating to the products of biotechnology are well understood; and
Indicate what scientific capabilities, tools, and expertise may be useful to the regulatory agencies to support oversight of potential future products of biotechnology.
Human drugs and medical devices were not included in the purview of the study.
To address its statement of task, the Committee gathered information from a number of sources, and heard from over 70 speakers over the course of three in-person meetings and eight webinars. The Committee received responses to a request for information from a dozen federal agencies, and solicited statements and written comments from members for the public. According to the report, the Committee defined biotechnology products as products developed through genetic engineering or genome engineering (including products where the engineered DNA molecule is itself the product, as in an engineered molecule used as a DNA information-storage medium) or the targeted or in vitro manipulation of genetic information of organisms, including plants, animals, and microbes. The term also covers some products produced by such plants, animals, microbes, and cell-free systems or products derived from all of the above.
The Committee grouped future products into three major classes:
Open-release products: The open-release products that the Committee saw on the horizon include plants, animals, microbes, and synthetic organisms that have been engineered for deliberate release in an open environment. According to the report, the ability to sustain existence in the environment with little or no human intervention is a key change between existing products of biotechnology and some of the future ones anticipated in this class. The report states that the Committee thought that future open-release products would be developed for familiar uses, such as agricultural crops, but would also likely be developed for uses such as cleaning up contaminated sites with engineered microbes, replacing animal-derived meat with meat cultured from animal cells, and controlling invasive species through gene drives;
Contained products: The Committee concluded that future biotechnology products that are produced in contained environments are more likely to be microbial based or synthetically based rather than based on an animal or plant host. According to the report, organisms of many genera are used in fermenters to produce commodity chemicals, fuels, specialty chemicals or intermediates, enzymes, polymers, food additives, and flavors. When considering the laboratory as a contained environment, the report states that many examples of transgenic animals from vendors are widely used today for research and development. Because performing biotechnology in contained environments allows higher control over the choice of host organism, systems with advanced molecular toolboxes are already in high use; and
Platforms: Biotechnology platforms are tools that are used in the creation of other biotechnology products, according to the report, including products that are traditionally characterized as wet lab, such as DNA/RNA, enzymes, vectors, cloning kits, cells, library prep kits, and sequencing prep kits, and products that are dry lab, such as vector drawing software, computer-aided design software, primer calculation software, and informatics tools. The report states that these two categories continue to meld as newer approaches are published or commercialized.
The report notes that there are a variety of technical, economic, and social trends that drive and will continue to drive the types of biotechnology products developed in the next decade. Technical and economic trends in the biological sciences and biological engineering are accelerating the rate at which new product ideas are formulated and the number of actors who are involved in product development. The report states that with regard to social trends, it was evident to the Committee that there are many competing interests, risks, and benefits regarding future biotechnology products. According to the report, it was clear that the U.S. and international regulatory systems will need to achieve a balance among these competing aspects when considering how to manage the development and use of new biotechnology products.
The Committee found that the Coordinated Framework appears to have considerable flexibility in statutory authority to cover a wide range of biotechnology products. The jurisdictions of EPA, FDA, and USDA are defined in ways that may leave gaps or redundancies in regulatory oversight, however. According to the report, even when jurisdiction exists, the available legal authorities may not be ideally tailored to new and emerging biotechnology products. Other agencies will likely have responsibilities to regulate some future biotechnology products, and their roles are not well specified in the Coordinated Framework.
The report states that the Committee found that the complexity of the existing biotechnology regulatory system, which could appear fragmented, results in a system that is difficult for product developers -- including individuals, nontraditional organizations, and small enterprises, as well as consumers, product users, and interested members of the public to navigate. The complexity can cause uncertainty and a lack of predictability for developers of future biotechnology products and creates the potential for loss of public confidence in oversight of future biotechnology products.
According to the report, the increased rate of new product ideas means that the types and number of biotechnology products in the next five to ten years may be significantly larger than the current rate of product introduction. The report cautions EPA, FDA, USDA, and other relevant agencies to prepare for this potential increase, including finding effective means of evaluation that maintains public safety, protects the environment, and satisfies the statutory requirements appropriate for each agency. The increased number of actors involved in product development means that the regulatory agencies will need to be prepared to provide information regarding the regulatory process to groups that may have little familiarity with the Coordinated Framework.
According to the report, advances in biotechnology are leading to products that involve the transformation of less familiar host organisms, have multiple engineered pathways, are comprised of DNA from multiple organisms, or are made from entirely synthetic DNA. Such products may have few or no comparators to existing nonbiotechnology products, which function as the baseline of comparison in current regulatory risk assessments of biotechnology products.
For future biotechnology products in all degrees of complexity and novelty, the Committee considered the risk assessment endpoints related to human health or environmental outcomes, such as illness, injury, death, or loss of ecosystems function. The Committee concluded that these endpoints are not new, but the intermediate steps along the path to those endpoints may be more complex, more ambiguous, and less well characterized than those for existing biotechnology products. According to the report, the scope, scale, complexity, and tempo of biotechnology products likely to enter the regulatory system in the next five to ten years have the potential to critically stress EPA, FDA, and USDA, both in terms of capacity and expertise.
At a high level, the Committee found that there are existing frameworks, tools, and processes for risk analyses and public engagement that can be used to address the issues likely to arise in future biotechnology products in a way that balances competing issues and concerns. Given the profusion of biotechnology products that are on the horizon, however, there is a risk that the capacity of the regulatory agencies may not be able to provide efficiently the quantity and quality of risk assessments that will be needed. The report states that an important approach for dealing with the increase in the products will be the increased use of stratified approaches to regulation, where new and potentially more complex risk analysis methods will need to be developed for some products, while established risk analysis methods can be applied or modified to address products that are familiar or that require less complex risk analysis. To help articulate what capabilities, tools, and expertise might be useful to meet these objectives, the Committee created a conceptual map for decision-making aimed to assess and manage product risk, streamline regulation requirements, and increase transparency.
The Committee identified the following broad themes regarding future opportunities for enhancement of the U.S. biotechnology regulatory system:
The bioeconomy is growing rapidly and the U.S. regulatory system needs to provide a balanced approach for consideration of the many competing interests in the face of this expansion;
The profusion of biotechnology products over the next five to ten years has the potential to overwhelm the U.S. regulatory system, which may be exacerbated by a disconnect between research in regulatory science and expected uses of future biotechnology products;
Regulators will face difficult challenges as they grapple with a broad array of new types of biotechnology products -- for example, cosmetics, toys, pets, and office supplies -- that go beyond contained industrial uses and traditional environmental release (for example, Bacillus thuringiensis (Bt) or herbicide-resistant crops);
The safe use of new biotechnology products requires rigorous, predictable, and transparent risk-analysis processes whose comprehensiveness, depth, and throughput mirror the scope, scale, complexity, and tempo of future biotechnology applications; and
In addition to the conclusions and recommendations from this report, EPA, FDA, USDA, and other agencies involved in regulation of future biotechnology products would benefit from adopting recommendations made by previous National Academies committees related to future products of biotechnology that are consistent with the findings and recommendations in this report.
On the basis of its conclusions, the Committee developed a number of detailed recommendations regarding actions that can be taken to enhance the capabilities of the biotechnology regulatory system to be prepared for anticipated future products of biotechnology.
EPA, FDA, USDA, and other agencies involved in regulation of future biotechnology products should increase scientific capabilities, tools, expertise, and horizon scanning in key areas of expected growth of biotechnology, including natural, regulatory, and social sciences;
EPA, FDA, and USDA should increase their use of pilot projects to advance understanding and use of ecological risk assessments and benefit analyses for future biotechnology products that are unfamiliar and complex and to prototype new approaches for iterative risk analyses that incorporate external peer review and public participation; and
The National Science Foundation, the Department of Defense, the Department of Energy, the National Institute of Standards and Technology, and other agencies that fund biotechnology research with the potential to lead to new biotechnology products should increase their investments in regulatory science and link research and education activities to regulatory-science activities.
The report is well written and contains a significant amount of new and valuable information on the types of new biotechnology products being innovated and coming into commerce, trends of note regarding future products, and regulatory gaps and redundancies that need to be addressed. This background information is clearly presented and supports well the conclusions that are essential to understand, and the recommendations that are in urgent need of response.
That the federal agencies tasked with regulating biotechnology products need increased funding and organizational retooling to address the challenges eloquently and convincingly described in the report are truths beyond dispute. In this political climate, and under this Administration, meeting these needs will be challenging. Shareholders of all sorts in the biotechnology area -- businesses, innovators, environmental and public health activists -- are urged to weigh in and express support for the allocation of resources needed to fulfill the reports recommendations. Future generations of biotechnology products are on the line and at risk if these recommendations fall on deaf ears.
2017 Bergeson & Campbell, P.C.
See the article here:
National Academies Report Finds Future Biotechnology Products May Overwhelm Agencies - The National Law Review
Posted in Biotechnology
Comments Off on National Academies Report Finds Future Biotechnology Products May Overwhelm Agencies – The National Law Review
Puma Biotechnology Inc (PBYI) Soars 11.86% on March 13 – Equities.com
Posted: March 14, 2017 at 2:48 pm
Market Summary Follow
Puma Biotechnology Inc is a A biopharmaceutical company
PBYI - Market Data & News
PBYI - Stock Valuation Report
Puma Biotechnology Inc (PBYI) had a good day on the market for Monday March 13 as shares jumped 11.86% to close at $44.80. About 1.88 million shares traded hands on 13,031 trades for the day, compared with an average daily volume of 972,852 shares out of a total float of 36.95 million. After opening the trading day at $40.05, shares of Puma Biotechnology Inc stayed within a range of $45.20 to $39.80.
With today's gains, Puma Biotechnology Inc now has a market cap of $1.66 billion. Shares of Puma Biotechnology Inc have been trading within a range of $73.27 and $19.74 over the last year, and it had a 50-day SMA of $34.81 and a 200-day SMA of $42.33.
Puma Biotechnology Inc is a biopharmaceutical company. It is engaged in the acquisition, development and commercialization of products to enhance cancer care.
Puma Biotechnology Inc is based out of Los Angeles, CA and has some 160 employees. Its CEO is Alan H. Auerbach.
For a complete fundamental analysis of Puma Biotechnology Inc, check out Equities.coms Stock Valuation Analysis report for PBYI.
Want to invest with the experts? Subscribe to Equities Premium newsletters today! Visit http://www.equitiespremium.com/ to learn more about Guild Investments Market Commentary and Adam Sarhans Find Leading Stocks today.
Puma Biotechnology Inc is also a component of the Russell 2000. The Russell 2000 is one of the leading indices tracking small-cap companies in the United States. It's maintained by Russell Investments, an industry leader in creating and maintaining indices, and consists of the smallest 2000 stocks from the broader Russell 3000 index.
Russell's indices differ from traditional indices like the Dow Jones Industrial Average (DJIA) or S&P 500, whose members are selected by committee, because they base membership entirely on an objective, rules based methodology. The 3,000 largest companies by market cap make up the Russell 3000, with the 2,000 smaller companies making up the Russell 2000. It's a simple approach that gives a broad, unbiased look at the small-cap market as a whole.
To get more information on Puma Biotechnology Inc and to follow the companys latest updates, you can visit the companys profile page here: PBYIs Profile. For more news on the financial markets and emerging growth companies, be sure to visit Equities.coms Newsdesk. Also, dont forget to sign-up for our daily email newsletter to ensure you dont miss out on any of our best stories.
All data provided by QuoteMedia and was accurate as of 4:30PM ET.
DISCLOSURE: The views and opinions expressed in this article are those of the authors, and do not represent the views of equities.com. Readers should not consider statements made by the author as formal recommendations and should consult their financial advisor before making any investment decisions. To read our full disclosure, please go to: http://www.equities.com/disclaimer
Read the original post:
Puma Biotechnology Inc (PBYI) Soars 11.86% on March 13 - Equities.com
Posted in Biotechnology
Comments Off on Puma Biotechnology Inc (PBYI) Soars 11.86% on March 13 – Equities.com
NAS Issues Report on Preparing for Future Products of Biotechnology: National Academies of Sciences, Engineering … – The National Law Review
Posted: March 13, 2017 at 8:44 pm
On March 9, 2017, the National Academies of Sciences, Engineering, and Medicine (NAS) announced the release (pre-publication version) of a new report:Preparing for Future Products of Biotechnology.Pursuant to the White House Office of Science and Technology Policy's (OSTP) July 2, 2015, memorandum, Modernizing the Regulatory System for Biotechnology Products, NAS was tasked with looking into the future and describing the possible future products of biotechnology that will arise over the next five to ten years, as well as providing some insights that can help shape the capabilities within the agencies as they move forward.
Via an ad hoc committee, the Committee on Future Biotechnology Products and Opportunities to Enhance Capabilities of the Biotechnology Regulatory System, NAS developed this report through several months of gathering and synthesizing information from several sources, including: 74 speakers over the course of three in-person meetings and eight webinars, including one presented by Lynn L. Bergeson; responses to its request for information from a dozen federal agencies; statements solicited from members of the public at its in-person meetings; written comments through the duration of the study; and recent NAS studies related to future products of biotechnology.
The report presents conclusions concerning the future biotechnology products themselves, as well the challenges that federal agencies will face in regulating them, which include:
The bioeconomy is growing rapidly and the U.S. regulatory system needs to provide a balanced approach for consideration of the many competing interests in the face of this expansion;
The profusion of biotechnology products over the next five to ten years has the potential to overwhelm the U.S. regulatory system, which may be exacerbated by a disconnect between research in regulatory science and expected uses of future biotechnology products;
Regulators will face difficult challenges as they grapple with a broad array of new types of bio-technology products -- for example, cosmetics, toys, pets, and office supplies -- that go beyond contained industrial uses and traditional environmental release;
The safe use of new biotechnology products requires rigorous, predictable, and transparent risk-analysis processes whose comprehensiveness, depth, and throughput mirror the scope, scale, complexity, and tempo of future biotechnology applications.
The report provides three recommendations for federal agencies in responding to these challenges, which it states should be taken to enhance the ability of the biotechnology regulatory system to oversee the consumer safety and environmental protection required for future biotechnology products:
The U.S. Environmental Protection Agency (EPA), the U.S. Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and other agencies involved in regulation of future bio-technology products should increase scientific capabilities, tools, expertise, and horizon scanning in key areas of expected growth of biotechnology, including natural, regulatory, and social sciences.
EPA, FDA, and USDA should increase their use of pilot projects to advance understanding and use of ecological risk assessments and benefit analyses for future biotechnology products that are unfamiliar and complex and to prototype new approaches for iterative risk analyses that incorporate external peer review and public participation.
The National Science Foundation, the Department of Defense, the Department of Energy, the National Institute of Standards and Technology, and other agencies that fund bio-technology research with the potential to lead to new biotechnology products should increase their investments in regulatory science and link research and education activities to regulatory-science activities.
The report is well-written and contains an impressive amount of new, relevant, and important information. The Committee participants are to be commended for an important new piece of scholarship in this area.
The reports conclusions are also significant, but not entirely unexpected.For those of us working in this space, we have recognized for years the lack of clarity regarding jurisdictional boundaries, the paucity of government resources, and the urgent need for regulatory clarity and significantly enhanced funding. Unfortunately, given current Trump Administration efforts to diminish government funding for EPA, FDA, and elsewhere, the well-crafted and spot-on recommendations may tragically fall on deaf ears.Shareholders should carefully review the report and work hard to ensure the recommendations are implemented. The consequences of failing to increase scientific capabilities, tools, expertise, and horizon scanning in key areas of expected growth of biotechnology, including natural regulatory, and social sciences -- the number one recommendation in the report -- are too great to ignore.
2017 Bergeson & Campbell, P.C.
See original here:
NAS Issues Report on Preparing for Future Products of Biotechnology: National Academies of Sciences, Engineering ... - The National Law Review
Posted in Biotechnology
Comments Off on NAS Issues Report on Preparing for Future Products of Biotechnology: National Academies of Sciences, Engineering … – The National Law Review
Portugal to support Nigeria in Biotechnology activities – TV360 Nigeria – TV360
Posted: March 13, 2017 at 8:44 pm
The Portuguese Minister of Science and Technology, Manuel Heitor, say his country will offer technical support to Nigeria in key biotechnology activities that will enhance tropical agriculture and food security.
Heitor made this known in a statement made available to the News Agency of Nigeria (NAN) by the Head, Protocol and Communications, National Biotechnology Development Agency (NABDA), Ifeoma Ndefo on Monday in Abuja.
The minister visited the Director-General, National Biotechnology Development Agency (NABDA), LucyOgbadu, with a team of experts in different fields of biotechnology from various institutions in Portugal.
In his address, Heitor indicated interest of the Portuguese delegation in partnering with the NABDA on efforts to expand technologies that would enhance tropical agriculture and food security.
The minister,who is also the minister of Education, declared the interest of his country in assisting NABDA scientific officers wishing to acquire Masters and P.HD degrees in Portugal.
He identified areas of NABDAs activities akin to their fields of competencessuch as cancer prevention, stem cell research, Biogas production (converting wastes to generate electricity) and bio-remediation.
Heitor said all of thesewere areas of interest for collaboration as well as knowledge sharing between their institutions and NABDA.
On her part, Director-General, NABDA, commended the minister and his team for their interest to partner with the Agency and to ensure that Nigeria reaps the potential benefit that biotechnology offers.
The NABDA boss highlighted the various projects and activities of the five technical departments of the Agency.
This included the six Centres of Excellence located at frontline universities of the each of the Six Geo-political Zones of the nation (Uni-Jos, ABU Zaria, Uni- Port, UNN, Uni-Ibadan and Uni- Maiduguri).
Others are the NABDAs Bioresources Centres (BioDecs) spread across twenty-five states of the federation.
The News Agency of Nigeria recalls that the agency was established by the Federal Government to implement policy aimed at promoting, coordinating, and setting research and development priority in biotechnology for Nigeria.
NAN.
The rest is here:
Portugal to support Nigeria in Biotechnology activities - TV360 Nigeria - TV360
Posted in Biotechnology
Comments Off on Portugal to support Nigeria in Biotechnology activities – TV360 Nigeria – TV360
Puma Biotechnology Inc (PBYI) Moves Higher on Volume Spike for March 13 – Equities.com
Posted: March 13, 2017 at 8:44 pm
Market Summary Follow
Puma Biotechnology Inc is a A biopharmaceutical company
PBYI - Market Data & News
PBYI - Stock Valuation Report
Puma Biotechnology Inc (PBYI) traded on unusually high volume on Mar. 13, as the stock gained 11.86% to close at $44.80. On the day, Puma Biotechnology Inc saw 1.88 million shares trade hands on 13,031 trades. Considering that the stock averages only a daily volume of 1.15 million shares a day over the last month, this represents a pretty significant bump in volume over the norm.
Generally speaking, when a stock experiences a sudden spike in trading volume, it may be seen as a bullish signal for investors. An increase in volume means more market awareness for the company, potentially setting up a more meaningful move in stock price. The added volume also provides a level of support and stability for price advances.
The stock has traded between $73.27 and $19.74 over the last 52-weeks, its 50-day SMA is now $34.81, and its 200-day SMA $42.33. Puma Biotechnology Inc has a P/B ratio of 7.89.
Puma Biotechnology Inc is a biopharmaceutical company. It is engaged in the acquisition, development and commercialization of products to enhance cancer care.
Headquartered in Los Angeles, CA, Puma Biotechnology Inc has 160 employees and is currently under the leadership of CEO Alan H. Auerbach.
For a complete fundamental analysis analysis of Puma Biotechnology Inc, check out Equities.coms Stock Valuation Analysis report for PBYI.
Want to invest with the experts? Subscribe to Equities Premium newsletters today! Visit http://www.equitiespremium.com/ to learn more about Guild Investments Market Commentary and Adam Sarhans Find Leading Stocks today.
To get more information on Puma Biotechnology Inc and to follow the companys latest updates, you can visit the companys profile page here: PBYIs Profile. For more news on the financial markets and emerging growth companies, be sure to visit Equities.coms Newsdesk. Also, dont forget to sign-up for our daily email newsletter to ensure you dont miss out on any of our best stories.
All data provided by QuoteMedia and was accurate as of 4:30PM ET.
DISCLOSURE: The views and opinions expressed in this article are those of the authors, and do not represent the views of equities.com. Readers should not consider statements made by the author as formal recommendations and should consult their financial advisor before making any investment decisions. To read our full disclosure, please go to: http://www.equities.com/disclaimer
The rest is here:
Puma Biotechnology Inc (PBYI) Moves Higher on Volume Spike for March 13 - Equities.com
Posted in Biotechnology
Comments Off on Puma Biotechnology Inc (PBYI) Moves Higher on Volume Spike for March 13 – Equities.com
Woodrose Ventures Corporation Announces Proposed Acquisition of Global Stem-Cell Biotechnology Company … – Marketwired (press release)
Posted: March 13, 2017 at 8:43 pm
VANCOUVER, BRITISH COLUMBIA--(Marketwired - March 13, 2017) -
NOT FOR DISSEMINATION IN THE UNITED STATES
Editors Note: There is a photo associated with this press release.
Woodrose Ventures Corporation (TSX VENTURE:WRS.H) ("Woodrose" or the "Company") is pleased to announce that it has entered into an agreement (the "Agreement") dated March 10, 2017 to acquire all of the shares of Novoheart Holdings Ltd. ("Novoheart"), a global stem cell biotechnology company dedicated to human heart engineering (the "Transaction"). Novoheart develops products and provides services focused on engineering prototypes of bio-artificial human heart tissues and chambers for drug discovery, cardiotoxicity screening, disease modelling and therapeutic applications.
The Transaction will constitute a "reverse-takeover" of Woodrose in accordance with the policies of the TSX Venture Exchange (the "TSXV") and the reactivation of Woodrose, which is currently a NEX-listed issuer.
About Novoheart
Novoheart is a global stem cell biotechnology company headquartered in Hong Kong with R&D Innovation Centres being set up in the United States. Novoheart's mission is to revolutionize drug discovery and the development of heart therapeutics with its range of proprietary bioengineered human heart constructs, collectively known as the MyHeart platform, and to further develop them into transplantable heart grafts for cell-based regenerative therapies with superior safety and efficacy. Its scientific team has pioneered a range of best-in-class bioengineering technologies and constructed the world's first human mini-heart "novoHeart" with which the Novoheart team intends to revolutionize:
1) Pre-clinical drug discovery, cardiotoxicity screening and heart disease modelling;
2) Post-discovery, clinical development of novel therapeutics; and
3) Pre-clinical and clinical development of cell-based cardiac regenerative therapies.
Novoheart's immediate focus is to innovate and accelerate the lengthy, expensive and inefficient drug development process. The development of a new drug candidate typically costs US$2-4bn and takes 10+ years (Tufts Centre for the Study of Drug Development, Tufts CSDD R&D Cost Study 2014) with extremely poor success rates of
Novoheart's intellectual property portfolio, including the human "heart-in-a-jar" (novoHeart) and other related next-generation technologies of the MyHeart platform (see figure below) are unique solutions that help bridge the gap between pre-clinical and clinical drug trials. The MyHeart platform provides advanced human heart surrogates for pre-screening of drug formulas and the elimination of toxic compounds early on in the drug development process, minimizing the risk towards patients. Significantly, the MyHeart Platform provides real time data on the effects of drug formulations enabling drug development companies to undertake "on-the-fly" reformulation of drug candidates to optimize efficacy and toxicological profiles. With Novoheart's technologies, we aim to significantly reduce pre-clinical R&D time and costs, and importantly, improve trial successes. It is anticipated that drug screening results using Novoheart's human engineered tissues would be accepted as reliable indicators for toxicity and efficacy, thereby qualifying the test compounds for accelerated drug development.
Novoheart adopts a hybrid business model by:
These products and services are designed to significantly reduce the time, cost, and use of animal models, as well as improve patient safety, and facilitate pharmaceutical discovery and development. Novoheart is currently working with leading academic and pharmaceutical partners to innovate drug discovery and toxicity screening protocols. Our targeted clients are pharmaceutical companies, government units, and research institutions.
Novoheart was incorporated in 2014 pursuant to the laws of British Virgin Islands (BVI) and its controlling shareholder is Medera Group Limited, a BVI entity. Novoheart has one wholly owned Hong Kong subsidiary "Novoheart Limited" ("Novoheart Hong Kong") which is the group operating entity.
Novoheart Hong Kong was incorporated in January 2014 by founder and CEO Prof. Ronald Li, with scientific co-founders Prof. Kevin Costa and Prof. Michelle Khine.
Novoheart's foundational technologies are the direct outcome of over 15 years of research effort supported by R&D investments amounting to approximately USD30MM. These research efforts, performed at Johns Hopkins University, Icahn School of Medicine at Mount Sinai, University of California Irvine, University of California Davis, and the University of Hong Kong by our scientific founders, have received major recognitions such as American Heart Association's Best Study of 2005, Ground-breaking Study of 2006, and Late-breaking Studies of 2002, 2003, 2005 and 2007, and the Spirit of Hong Kong Innovating for Good Award in 2015. The "human-heart-in-a-jar" technology was selected by Google's Solve For X as a Moonshot Project in 2015.
Novoheart's scientific founders and advisors are renowned pioneering leaders in the stem cell and cardiac space, with a successful track record in developing and commercializing ground-breaking technologies. In September 2014, Novoheart established its R&D base and office in the Hong Kong Science Park, where it continues to innovate solutions for drug discovery and human heart tissue engineering.
In December 2014, Novoheart signed a strategic partnership with a major global pharmaceutical company (the "Global Pharma Partner") headquartered in New York City to validate the MyHeart platform. The success of this validation process has resulted in follow on income-generating projects.
In January 2015, Novoheart's R&D proposal to develop bio-artificial heart tissues for drug screening received 50/50 matched funding from the Innovation & Technology Commission (ITC) of the Government of Hong Kong, with a total project cost of over HK$21MM over 2 years. It was also the largest biotech project granted by ITC for that year. Novoheart owns all of the intellectual property generated from this project, and as a result of the R&D, Novoheart has applied or is in the application process for 3 new patents covering newly developed technology, including the human ventricular cardiac anisotropic sheet (hvCAS) as a powerful tool for detecting drug-induced arrhythmias with the results published in the prestigious international peer-reviewed bioengineering journal Advanced Materials (Shum et al. 2017, Advanced Materials, 29). Additionally, Novoheart holds exclusive worldwide licenses or options to acquire the same for technologies that constitute its MyHeart platform and future developments.
In December 2015, Novoheart signed a second contract with the Global Pharma Partner to build disease-specific engineered human heart tissues and chambers for drug discovery. The total project cost is US$726,000 over 1.5 years.
In February 2017, the Corporate Venture Fund (CVF) of the Hong Kong Science and Technology Parks Corporation (HKSTPC) completed an equity investment of approximately US$250,000 into Novoheart and an additional investment would be made at the Transaction.
Novoheart Financial Information
The following table includes a summary of certain financial information of Novoheart and is derived from its financial statements for the years ended June 30, 2016 and June 30, 2015.
Summary of the Transaction
Under the terms of the Agreement, the shareholders of Novoheart will receive an aggregate of 66,086,600 common shares of Woodrose on a post-Consolidation basis (see below) ("Woodrose Post-Consolidation Shares"). In addition, a finder's fee of 2,313,038 Woodrose Post-Consolidation Shares will be paid to Cynosure Private Equity Limited in connection with the Transaction.
In connection with the Transaction, Woodrose intends to complete a consolidation of all its outstanding common shares on the basis of 3.56878449 old common shares for each one new common share (the "Consolidation"). In addition, Woodrose intends to complete a non-brokered private placement (the "Private Placement") of 11,700,000 subscription receipts ("Subscription Receipts") at a price of CDN$0.50 per Subscription Receipt to raise gross proceeds of CDN$5,850,000, which will be held in escrow in accordance with the terms of a subscription receipt agreement (the "Subscription Receipt Agreement"). It is anticipated that the Subscription Receipt Agreement will provide that, upon completion of the Transaction, each Subscription Receipt will automatically convert into one Woodrose Post-Consolidation Share. The Subscription Receipt Agreement will also provide that, in the event the Transaction is terminated or does not complete within an agreed timeframe, the Subscription Receipts will be cancelled and the funds will be returned to the holders. Woodrose may pay cash fees in an amount not to exceed 7% of the gross proceeds (to a maximum of $364,000) to certain finders involved in the Private Placement and may issue finder's warrants ("Finder's Warrants"), in an amount not to exceed 7% of the number of Subscription Receipts issued (to a maximum of 728,000 Finders Warrants) each of which would entitle the holder to acquire one Woodrose Post-Consolidation Share at a price of CDN$0.50 for a period of two years following closing of the Private Placement. All securities issued pursuant to the Private Placement will be subject to a statutory hold period of four months and one day.
The Company intends to use the net proceeds of the offering to finance investment in drug discovery and screening, establish commercial partnerships, expand the current laboratory, hire additional research and development team members and for working capital and general corporate purposes.
Upon completion of the Transaction, it is anticipated that the Company will be classified as a Tier 2 Technology Issuer on the TSXV and will change its name to "Novoheart Holdings (BC) Limited" or such other name as is acceptable to the Board of Directors. Closing of the Transaction ("Closing") is subject to conditions precedent, that include, but are not limited to, the following:
The Transaction is an "arm's length" transaction (as defined by the policies of the TSXV). Woodrose intends to rely an exemption from the sponsorship requirements of the policies of the TSXV.
Proposed Management Team
Upon closing of the Transaction, the following directors and senior officers are anticipated to be appointed in replacement of Woodrose's current board and management:
Prof. Ronald Li, B.Sc. (Hons), Ph.D. (Proposed President, Chief Executive Officer and Director)
Prof. Ronald Li is a co-founder of Novoheart, and has been serving as the CEO since 2016. He is concurrently Director of Ming-Wai Lau Centre for Reparative Medicine, HK node, Karolinska Institutet (KI), Sweden, with a professorial cross appointment at the Dr. Li Dak-Sum Research Centre, The University of Hong Kong (HKU)-KI Collaboration in Regenerative Medicine of HKU. Prof. Li has been an advocate of stem cell technology for many years, starting from his career as Assistant Professor of Cardiology, and Cellular and Molecular Medicine at the Johns Hopkins University (JHU) School of Medicine. He founded and led the Human Embryonic Stem Cell Consortium when he was recruited in 2005 to become a tenured Associate Professor at the University of California, Davis, in light of state's USD3-billion stem cell initiative Proposition 71. Prof. Li was the Founding Director of the Stem Cell & Regenerative Medicine Consortium (SCRMC) at the University of Hong Kong (HKU) from 2010 to 2015. He also co-directed the Section of Cardiovascular Cell & Tissue Engineering in Icahn School of Medicine at Mount Sinai with Prof. Kevin Costa. Prof. Li has received multiple accolades and recognitions during his career, including the Spirit of Hong Kong Innovating for Good Award by the South China Morning Post (2015), the Top Young Faculty Award (2002, 2004), the Top Prize for the Young Investigator Basic Research (2001) and Top Postdoctoral Fellow Helen Taussig Award (2001) of JHU School of Medicine, Young Investigator Award 1st Prize from the Heart Rhythm Society (2002), and the Career Development Award from the Cardiac Arrhythmias Research & Education Foundation (2001).
Prof. Li graduated with his B.S. with honors in Biotechnology from University of Waterloo, Ontario, on Dean's List and his Ph.D. in Cardiology/Physiology at the University of Toronto.
Dr. Camie Chan, B.Sc. (Hons), M.Sc., Ph.D. (Proposed Chief Operating Officer and Director)
Dr. Camie Chan joined Novoheart Hong Kong as the Chief Operating Officer in 2016, after having served at HKU as the Deputy Director of the Faculty of Medicine Core Facility, a founding member of the Management Committee of the Stem Cell & Regenerative Medicine Consortium (SCRMC), and Assistant Professor in the Department of Anatomy, between 2010 and 2016. She has had extensive experience managing laboratory operations in her capacity at HKU, and her prior career as Assistant Professor at the University of California, Davis, and Assistant Investigator at the Shriners Hospital for Children. Dr. Chan is also a co-inventor of technology allowing mass production of human ventricular heart cells from pluripotent stem cells.
Dr. Chan graduated with her B.Sc. with honors at the University of Waterloo, followed by obtaining her M.Sc. degree in Medical Sciences and Ph.D. degree in Immunology at the University of Toronto, Canada. She then received postdoctoral training at the Sydney Kimmel Cancer Research Center at the Johns Hopkins University. She has garnered numerous awards in her career, including the prestigious National Institute of Allergy and Infectious Diseases (NIAID) Developmental Research Grant Award.
Prof. Kevin Costa, B.S., Ph.D. (Proposed Chief Scientific Officer)
Prof. Costa is Director of the Section of Cardiovascular Cell and Tissue Engineering at the Icahn School of Medicine at Mount Sinai in New York City. Prof. Costa was previously trained at the Johns Hopkins University and on the faculty as Associate Professor of Biomedical Engineering at Columbia University. As a "blue-blood" biomedical engineering (BME) expert (B.S. and M.S. in BME from Boston University, Ph.D. in BME from UC San Diego, and postdoc in BME from JHU and University of Washington) in cell and tissue biomechanics and cardiac tissue engineering, he has developed one of the first engineered cardiac tissue systems. Since 2009, he has been working with Prof. Ronald Li to translate such systems into human cells. Prof. Costa has received research funding from the Whitaker Foundation, the National Science Foundation (NSF) and the National Institutes of Health (NIH; NHLBI, NIBIB, and NIGMS). He was also a recipient of the prestigious Faculty Early Career Development (CAREER) Award from the NSF. Prof. Costa is an inventor of several cardiac tissue engineering technologies and one of the scientific co-founders of Novoheart Hong Kong.
Ms. Iris Lo, B. Comm. (Hons), CPA, CA (Proposed Chief Financial Officer)
Ms. Lo is a seasoned professional with expertise in corporate finance, mergers and acquisitions, accounting, and finance. Prior to joining Novoheart, Ms. Lo was the Director of Corporate Development & Analysis at Cardiome Pharma Corp., a Canadian public company dually listed on the TSX and NASDAQ (TSX: COM, NASDAQ: CRME). At Cardiome, she held responsibilities in equity and debt financing, corporate mergers and acquisitions, product licensing and distributions, financial planning and analysis, as well as regulatory and risk management. During her tenure at Cardiome, Ms. Lo participated in transactions totaling over US$240 million as Cardiome grew from a company with a market capitalization of US$25 million to over US$150 million at its peak. She brings with her valuable experience from the life sciences and pharmaceutical sector, as well as expertise in dealing with the complexities of operating and financing public corporations. Ms. Lo was also previously a Manager in the Transaction Services team at PwC Hong Kong and began her career articling with KPMG Vancouver. She is a Chartered Professional Accountant and holds a Bachelor of Commerce (Honours) from the Sauder School of Business at the University of British Columbia.
Mr. Victor Chang (Proposed Director)
Mr. Chang is a seasoned investor who has lately become focused on start-ups. Mr. Chang started his career with Lippo Securities Limited in 1996 and became a Director of Grand International Holdings Limited in 1999, which was engaged in general investments. During the period from 2007 to 2009, he was a Director and Responsible Officer for Astrum Capital Management Limited carrying out regulated activities under the Securities and Futures Ordinance ("SFO", Cap. 571, Laws of Hong Kong) and with Murtsa Capital Partners Limited as well. During the period from 2007 to 2012, he was also a compliance consultant for Astrum Capital Management Limited. As co-founder and Managing Director of Zebra Strategic Outsource Solution, he has over 16 years of experience in recruitment process outsourcing, executive search as well as and private investment management. In Apil 2013, he successfully brought Zebra Strategic Holdings Limited which offers holistic HR solutions to IPO on the HK GEM board (Stock Code: 8260) and was re-designated as and is currently a Non-Executive Director with the company. He is currently a Director and Responsible Officer of Dakin Financial Group, a corporation licensed to carry out type 1, 2 & 9 regulated activity under the Hong Kong Securities and Futures Ordinance.
Mr. Tong Ricky Chiu (Proposed Director)
As a key founder and visionary for Grand Power Logistics Group Inc., which was listed on the TSX Venture Exchange (GPW.V) before its privatization in 2016, and Baoshinn International Express Ltd., Mr. Chiu adds value with his immense corporate development and growth skills. He received his education in Oxford University, England, and Beijing University, and began his career in Australia. He has a diversified background in a wide range of industries with roles in finance, audit, real estate, merchandise trading and travel, as well as logistics.
Mr. James Topham (Proposed Director)
Mr. Topham is an experienced executive with expertise in finance, accounting, auditing and entrepreneurial technology companies. He was an audit partner leading KPMG's Technology Group in the Vancouver office for 20 years where he worked with many fast growing public companies and was involved in many M&A and IPO transactions in Canada, the US and Europe. Mr. Topham founded Social Venture Partners Vancouver in 2001 with a mission to strengthen the organizational capacity of innovative non-profits serving children in-need and youth at-risk. It has funded several million dollars and provided thousands of hours of executive time mentoring these local non-profits. Since retiring at KPMG 7 years ago, Mr. Topham has worked on several Boards of both public and private technology companies. He received a lifetime achievement award from the BC Technology Industry Association and was awarded the designation of Fellow Chartered Public Accountant (FCPA) from the Chartered Public Accountants of BC for his career achievements in the profession and community. He was a founder and Board member for 9 years of the BC Technology Industry Association that represents the technology industry in BC. Mr. Topham is a CPA and has a Bachelor of Commerce degree with Honours from the University of Saskatchewan graduating as the most distinguished graduate in the College of Commerce.
Mr. Allen Ma (Proposed Director)
As a 30-year technology industry veteran, Mr. Ma was the CEO of Hong Kong Science & Technology Parks before he retired in July 2016. He held senior executive positions within the information and communications technology sector. His past roles include president for Asia-Pacific at British Telecom, vice-president for Asia at the global telecom solutions sector of Motorola, executive director of Hong Kong Telecommunications - subsequently called Cable & Wireless HKT - and managing director of Hong Kong Telecom CSL. Ma holds an MBA from the University of Toronto and is a fellow member of both the Chartered Institute of Management Accountants, UK and the Association of Chartered Certified Accountants, UK. He is also a Certified Management Accountant of Canada.
Proposed Advisory Team
Novoheart is supported by a Scientific Advisory Board whose proposed composition consists of eminent scientists renowned in the fields of stem cells, cardiac biology and physiology, tissue engineering, and clinical cardiology including clinical trials research, from top academic research institutes in the U.S.A. Their technical expertise will guide the development of Novoheart as a forerunner in the application of cutting-edge technologies to develop new and better treatments for heart disease and beyond.
Further Details
Both the Company and Novoheart intend to work diligently to complete the conditions precedent to Closing and anticipate completion of the Transaction in the second quarter of 2017. The Company will update its shareholders with further details as they become available.
ON BEHALF OF WOODROSE VENTURES CORPORATION
Darren Devine, President, CEO and Director
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Completion of the Transaction is subject to a number of conditions, including but not limited to, Exchange acceptance and if applicable pursuant to Exchange requirements, majority shareholder approval. Where applicable, the Transaction cannot close until the required shareholder approval is obtained. There can be no assurance that the Transaction will be completed as proposed or at all.
Investors are cautioned that, except as disclosed in the Filing Statement to be prepared in connection with the Transaction, any information with respect to the Transaction may not be accurate or complete and should not be relied on. Trading in securities of the Company should be considered highly speculative.
The TSX Venture Exchange has in no way passed upon the merits of the Transaction and has neither approved nor disproved the contents of this news release.
Cautionary Note Regarding Forward-Looking Statements
Information set forth in this news release may involve forward-looking statements under applicable securities laws. Forward-looking statements are statements that relate to future, not past, events. In this context, forward-looking statements often address expected future business and financial performance, and often contain words such as "anticipate", "believe", "plan", "estimate", "expect", and "intend", statements that an action or event "may", "might", "could", "should", or "will" be taken or occur, or other similar expressions. All statements, other than statements of historical fact, included herein including, without limitation; statements about the terms and completion of the Transaction are forward-looking statements. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the following risks: failure to satisfy all conditions precedent to the Transaction, including shareholder approval, approval of the TSX Venture Exchange and completion of the necessary financings and the additional risks identified in the management discussion and analysis section of Woodrose Corporation's interim and most recent annual financial statement or other reports and filings with the TSX Venture Exchange and applicable Canadian securities regulators. Forward-looking statements are made based on management's beliefs, estimates and opinions on the date that statements are made and the respective companies undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change, except as required by applicable securities laws. Investors are cautioned against attributing undue certainty to forward-looking statements.
To view the photo associated with this press release, please visit the following link: http://www.marketwire.com/library/20170312-1088577_MyHeart_800.jpg
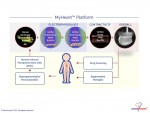
Originally posted here:
Woodrose Ventures Corporation Announces Proposed Acquisition of Global Stem-Cell Biotechnology Company ... - Marketwired (press release)
Posted in Biotechnology
Comments Off on Woodrose Ventures Corporation Announces Proposed Acquisition of Global Stem-Cell Biotechnology Company … – Marketwired (press release)
Puma Biotechnology Inc (NYSE:PBYI) Impact Score At 75 – Stock Observer
Posted: March 12, 2017 at 6:43 pm
Puma Biotechnology Inc (NYSE:PBYI) daily sentiment score is 0.103 for articles printed on 2017-03-10. This is on a -1-1 scale after assessing the buzzing news and its impact on the system. The assessment is hooked on the dependable web sources.
Bullish target is at $89 while conservative price target is $72 on respective equity.
Puma Biotechnology Inc has an ABR of 1.67. The stock rating was 1.67 in preceding quarter. Financial numbers can be released on 2017-05-09. Consensus estimated EPS is $-1.88 for this quarter.
Securities prices are motivated by fundamentals in both the controlled and open street. Informed or not, shareholders weightage is on fiscals and other allied valuation basics. The metrics under contemplation are per-share earnings and associated ratios. Investors shift their emphasis on comprehensive financial report. Also, they predict miscellaneous components, which consists the reserves and other firm resources and also its valuation to due debt. Definitely, it is an unusual exercise to assess and measure all features while generating funds in equity market. A disciplined appraisal bodes well when the notion is to contribute a fair part in planned income.
Whatever ratings Zacks gives can to some degree exhibit variance from calls of First Call. Both the entities dont poll same set of street analysts, and as a result the projections vary. Puma Biotechnology Inc (NYSE:PBYI) posted EPS of $-2.04 for period closed on 2016-12-31. The reported number was $-0.02 off from the consensus. In percentage terms variance was -0.99%.
Continue reading here:
Puma Biotechnology Inc (NYSE:PBYI) Impact Score At 75 - Stock Observer
Posted in Biotechnology
Comments Off on Puma Biotechnology Inc (NYSE:PBYI) Impact Score At 75 – Stock Observer
