VANCOUVER, BRITISH COLUMBIA--(Marketwired - March 13, 2017) -
NOT FOR DISSEMINATION IN THE UNITED STATES
Editors Note: There is a photo associated with this press release.
Woodrose Ventures Corporation (TSX VENTURE:WRS.H) ("Woodrose" or the "Company") is pleased to announce that it has entered into an agreement (the "Agreement") dated March 10, 2017 to acquire all of the shares of Novoheart Holdings Ltd. ("Novoheart"), a global stem cell biotechnology company dedicated to human heart engineering (the "Transaction"). Novoheart develops products and provides services focused on engineering prototypes of bio-artificial human heart tissues and chambers for drug discovery, cardiotoxicity screening, disease modelling and therapeutic applications.
The Transaction will constitute a "reverse-takeover" of Woodrose in accordance with the policies of the TSX Venture Exchange (the "TSXV") and the reactivation of Woodrose, which is currently a NEX-listed issuer.
About Novoheart
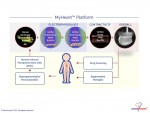
Novoheart is a global stem cell biotechnology company headquartered in Hong Kong with R&D Innovation Centres being set up in the United States. Novoheart's mission is to revolutionize drug discovery and the development of heart therapeutics with its range of proprietary bioengineered human heart constructs, collectively known as the MyHeart platform, and to further develop them into transplantable heart grafts for cell-based regenerative therapies with superior safety and efficacy. Its scientific team has pioneered a range of best-in-class bioengineering technologies and constructed the world's first human mini-heart "novoHeart" with which the Novoheart team intends to revolutionize:
1) Pre-clinical drug discovery, cardiotoxicity screening and heart disease modelling;
2) Post-discovery, clinical development of novel therapeutics; and
3) Pre-clinical and clinical development of cell-based cardiac regenerative therapies.
Novoheart's immediate focus is to innovate and accelerate the lengthy, expensive and inefficient drug development process. The development of a new drug candidate typically costs US$2-4bn and takes 10+ years (Tufts Centre for the Study of Drug Development, Tufts CSDD R&D Cost Study 2014) with extremely poor success rates of
Novoheart's intellectual property portfolio, including the human "heart-in-a-jar" (novoHeart) and other related next-generation technologies of the MyHeart platform (see figure below) are unique solutions that help bridge the gap between pre-clinical and clinical drug trials. The MyHeart platform provides advanced human heart surrogates for pre-screening of drug formulas and the elimination of toxic compounds early on in the drug development process, minimizing the risk towards patients. Significantly, the MyHeart Platform provides real time data on the effects of drug formulations enabling drug development companies to undertake "on-the-fly" reformulation of drug candidates to optimize efficacy and toxicological profiles. With Novoheart's technologies, we aim to significantly reduce pre-clinical R&D time and costs, and importantly, improve trial successes. It is anticipated that drug screening results using Novoheart's human engineered tissues would be accepted as reliable indicators for toxicity and efficacy, thereby qualifying the test compounds for accelerated drug development.
Novoheart adopts a hybrid business model by:
These products and services are designed to significantly reduce the time, cost, and use of animal models, as well as improve patient safety, and facilitate pharmaceutical discovery and development. Novoheart is currently working with leading academic and pharmaceutical partners to innovate drug discovery and toxicity screening protocols. Our targeted clients are pharmaceutical companies, government units, and research institutions.
Novoheart was incorporated in 2014 pursuant to the laws of British Virgin Islands (BVI) and its controlling shareholder is Medera Group Limited, a BVI entity. Novoheart has one wholly owned Hong Kong subsidiary "Novoheart Limited" ("Novoheart Hong Kong") which is the group operating entity.
Novoheart Hong Kong was incorporated in January 2014 by founder and CEO Prof. Ronald Li, with scientific co-founders Prof. Kevin Costa and Prof. Michelle Khine.
Novoheart's foundational technologies are the direct outcome of over 15 years of research effort supported by R&D investments amounting to approximately USD30MM. These research efforts, performed at Johns Hopkins University, Icahn School of Medicine at Mount Sinai, University of California Irvine, University of California Davis, and the University of Hong Kong by our scientific founders, have received major recognitions such as American Heart Association's Best Study of 2005, Ground-breaking Study of 2006, and Late-breaking Studies of 2002, 2003, 2005 and 2007, and the Spirit of Hong Kong Innovating for Good Award in 2015. The "human-heart-in-a-jar" technology was selected by Google's Solve For X as a Moonshot Project in 2015.
Novoheart's scientific founders and advisors are renowned pioneering leaders in the stem cell and cardiac space, with a successful track record in developing and commercializing ground-breaking technologies. In September 2014, Novoheart established its R&D base and office in the Hong Kong Science Park, where it continues to innovate solutions for drug discovery and human heart tissue engineering.
In December 2014, Novoheart signed a strategic partnership with a major global pharmaceutical company (the "Global Pharma Partner") headquartered in New York City to validate the MyHeart platform. The success of this validation process has resulted in follow on income-generating projects.
In January 2015, Novoheart's R&D proposal to develop bio-artificial heart tissues for drug screening received 50/50 matched funding from the Innovation & Technology Commission (ITC) of the Government of Hong Kong, with a total project cost of over HK$21MM over 2 years. It was also the largest biotech project granted by ITC for that year. Novoheart owns all of the intellectual property generated from this project, and as a result of the R&D, Novoheart has applied or is in the application process for 3 new patents covering newly developed technology, including the human ventricular cardiac anisotropic sheet (hvCAS) as a powerful tool for detecting drug-induced arrhythmias with the results published in the prestigious international peer-reviewed bioengineering journal Advanced Materials (Shum et al. 2017, Advanced Materials, 29). Additionally, Novoheart holds exclusive worldwide licenses or options to acquire the same for technologies that constitute its MyHeart platform and future developments.
In December 2015, Novoheart signed a second contract with the Global Pharma Partner to build disease-specific engineered human heart tissues and chambers for drug discovery. The total project cost is US$726,000 over 1.5 years.
In February 2017, the Corporate Venture Fund (CVF) of the Hong Kong Science and Technology Parks Corporation (HKSTPC) completed an equity investment of approximately US$250,000 into Novoheart and an additional investment would be made at the Transaction.
Novoheart Financial Information
The following table includes a summary of certain financial information of Novoheart and is derived from its financial statements for the years ended June 30, 2016 and June 30, 2015.
Summary of the Transaction
Under the terms of the Agreement, the shareholders of Novoheart will receive an aggregate of 66,086,600 common shares of Woodrose on a post-Consolidation basis (see below) ("Woodrose Post-Consolidation Shares"). In addition, a finder's fee of 2,313,038 Woodrose Post-Consolidation Shares will be paid to Cynosure Private Equity Limited in connection with the Transaction.
In connection with the Transaction, Woodrose intends to complete a consolidation of all its outstanding common shares on the basis of 3.56878449 old common shares for each one new common share (the "Consolidation"). In addition, Woodrose intends to complete a non-brokered private placement (the "Private Placement") of 11,700,000 subscription receipts ("Subscription Receipts") at a price of CDN$0.50 per Subscription Receipt to raise gross proceeds of CDN$5,850,000, which will be held in escrow in accordance with the terms of a subscription receipt agreement (the "Subscription Receipt Agreement"). It is anticipated that the Subscription Receipt Agreement will provide that, upon completion of the Transaction, each Subscription Receipt will automatically convert into one Woodrose Post-Consolidation Share. The Subscription Receipt Agreement will also provide that, in the event the Transaction is terminated or does not complete within an agreed timeframe, the Subscription Receipts will be cancelled and the funds will be returned to the holders. Woodrose may pay cash fees in an amount not to exceed 7% of the gross proceeds (to a maximum of $364,000) to certain finders involved in the Private Placement and may issue finder's warrants ("Finder's Warrants"), in an amount not to exceed 7% of the number of Subscription Receipts issued (to a maximum of 728,000 Finders Warrants) each of which would entitle the holder to acquire one Woodrose Post-Consolidation Share at a price of CDN$0.50 for a period of two years following closing of the Private Placement. All securities issued pursuant to the Private Placement will be subject to a statutory hold period of four months and one day.
The Company intends to use the net proceeds of the offering to finance investment in drug discovery and screening, establish commercial partnerships, expand the current laboratory, hire additional research and development team members and for working capital and general corporate purposes.
Upon completion of the Transaction, it is anticipated that the Company will be classified as a Tier 2 Technology Issuer on the TSXV and will change its name to "Novoheart Holdings (BC) Limited" or such other name as is acceptable to the Board of Directors. Closing of the Transaction ("Closing") is subject to conditions precedent, that include, but are not limited to, the following:
The Transaction is an "arm's length" transaction (as defined by the policies of the TSXV). Woodrose intends to rely an exemption from the sponsorship requirements of the policies of the TSXV.
Proposed Management Team
Upon closing of the Transaction, the following directors and senior officers are anticipated to be appointed in replacement of Woodrose's current board and management:
Prof. Ronald Li, B.Sc. (Hons), Ph.D. (Proposed President, Chief Executive Officer and Director)
Prof. Ronald Li is a co-founder of Novoheart, and has been serving as the CEO since 2016. He is concurrently Director of Ming-Wai Lau Centre for Reparative Medicine, HK node, Karolinska Institutet (KI), Sweden, with a professorial cross appointment at the Dr. Li Dak-Sum Research Centre, The University of Hong Kong (HKU)-KI Collaboration in Regenerative Medicine of HKU. Prof. Li has been an advocate of stem cell technology for many years, starting from his career as Assistant Professor of Cardiology, and Cellular and Molecular Medicine at the Johns Hopkins University (JHU) School of Medicine. He founded and led the Human Embryonic Stem Cell Consortium when he was recruited in 2005 to become a tenured Associate Professor at the University of California, Davis, in light of state's USD3-billion stem cell initiative Proposition 71. Prof. Li was the Founding Director of the Stem Cell & Regenerative Medicine Consortium (SCRMC) at the University of Hong Kong (HKU) from 2010 to 2015. He also co-directed the Section of Cardiovascular Cell & Tissue Engineering in Icahn School of Medicine at Mount Sinai with Prof. Kevin Costa. Prof. Li has received multiple accolades and recognitions during his career, including the Spirit of Hong Kong Innovating for Good Award by the South China Morning Post (2015), the Top Young Faculty Award (2002, 2004), the Top Prize for the Young Investigator Basic Research (2001) and Top Postdoctoral Fellow Helen Taussig Award (2001) of JHU School of Medicine, Young Investigator Award 1st Prize from the Heart Rhythm Society (2002), and the Career Development Award from the Cardiac Arrhythmias Research & Education Foundation (2001).
Prof. Li graduated with his B.S. with honors in Biotechnology from University of Waterloo, Ontario, on Dean's List and his Ph.D. in Cardiology/Physiology at the University of Toronto.
Dr. Camie Chan, B.Sc. (Hons), M.Sc., Ph.D. (Proposed Chief Operating Officer and Director)
Dr. Camie Chan joined Novoheart Hong Kong as the Chief Operating Officer in 2016, after having served at HKU as the Deputy Director of the Faculty of Medicine Core Facility, a founding member of the Management Committee of the Stem Cell & Regenerative Medicine Consortium (SCRMC), and Assistant Professor in the Department of Anatomy, between 2010 and 2016. She has had extensive experience managing laboratory operations in her capacity at HKU, and her prior career as Assistant Professor at the University of California, Davis, and Assistant Investigator at the Shriners Hospital for Children. Dr. Chan is also a co-inventor of technology allowing mass production of human ventricular heart cells from pluripotent stem cells.
Dr. Chan graduated with her B.Sc. with honors at the University of Waterloo, followed by obtaining her M.Sc. degree in Medical Sciences and Ph.D. degree in Immunology at the University of Toronto, Canada. She then received postdoctoral training at the Sydney Kimmel Cancer Research Center at the Johns Hopkins University. She has garnered numerous awards in her career, including the prestigious National Institute of Allergy and Infectious Diseases (NIAID) Developmental Research Grant Award.
Prof. Kevin Costa, B.S., Ph.D. (Proposed Chief Scientific Officer)
Prof. Costa is Director of the Section of Cardiovascular Cell and Tissue Engineering at the Icahn School of Medicine at Mount Sinai in New York City. Prof. Costa was previously trained at the Johns Hopkins University and on the faculty as Associate Professor of Biomedical Engineering at Columbia University. As a "blue-blood" biomedical engineering (BME) expert (B.S. and M.S. in BME from Boston University, Ph.D. in BME from UC San Diego, and postdoc in BME from JHU and University of Washington) in cell and tissue biomechanics and cardiac tissue engineering, he has developed one of the first engineered cardiac tissue systems. Since 2009, he has been working with Prof. Ronald Li to translate such systems into human cells. Prof. Costa has received research funding from the Whitaker Foundation, the National Science Foundation (NSF) and the National Institutes of Health (NIH; NHLBI, NIBIB, and NIGMS). He was also a recipient of the prestigious Faculty Early Career Development (CAREER) Award from the NSF. Prof. Costa is an inventor of several cardiac tissue engineering technologies and one of the scientific co-founders of Novoheart Hong Kong.
Ms. Iris Lo, B. Comm. (Hons), CPA, CA (Proposed Chief Financial Officer)
Ms. Lo is a seasoned professional with expertise in corporate finance, mergers and acquisitions, accounting, and finance. Prior to joining Novoheart, Ms. Lo was the Director of Corporate Development & Analysis at Cardiome Pharma Corp., a Canadian public company dually listed on the TSX and NASDAQ (TSX: COM, NASDAQ: CRME). At Cardiome, she held responsibilities in equity and debt financing, corporate mergers and acquisitions, product licensing and distributions, financial planning and analysis, as well as regulatory and risk management. During her tenure at Cardiome, Ms. Lo participated in transactions totaling over US$240 million as Cardiome grew from a company with a market capitalization of US$25 million to over US$150 million at its peak. She brings with her valuable experience from the life sciences and pharmaceutical sector, as well as expertise in dealing with the complexities of operating and financing public corporations. Ms. Lo was also previously a Manager in the Transaction Services team at PwC Hong Kong and began her career articling with KPMG Vancouver. She is a Chartered Professional Accountant and holds a Bachelor of Commerce (Honours) from the Sauder School of Business at the University of British Columbia.
Mr. Victor Chang (Proposed Director)
Mr. Chang is a seasoned investor who has lately become focused on start-ups. Mr. Chang started his career with Lippo Securities Limited in 1996 and became a Director of Grand International Holdings Limited in 1999, which was engaged in general investments. During the period from 2007 to 2009, he was a Director and Responsible Officer for Astrum Capital Management Limited carrying out regulated activities under the Securities and Futures Ordinance ("SFO", Cap. 571, Laws of Hong Kong) and with Murtsa Capital Partners Limited as well. During the period from 2007 to 2012, he was also a compliance consultant for Astrum Capital Management Limited. As co-founder and Managing Director of Zebra Strategic Outsource Solution, he has over 16 years of experience in recruitment process outsourcing, executive search as well as and private investment management. In Apil 2013, he successfully brought Zebra Strategic Holdings Limited which offers holistic HR solutions to IPO on the HK GEM board (Stock Code: 8260) and was re-designated as and is currently a Non-Executive Director with the company. He is currently a Director and Responsible Officer of Dakin Financial Group, a corporation licensed to carry out type 1, 2 & 9 regulated activity under the Hong Kong Securities and Futures Ordinance.
Mr. Tong Ricky Chiu (Proposed Director)
As a key founder and visionary for Grand Power Logistics Group Inc., which was listed on the TSX Venture Exchange (GPW.V) before its privatization in 2016, and Baoshinn International Express Ltd., Mr. Chiu adds value with his immense corporate development and growth skills. He received his education in Oxford University, England, and Beijing University, and began his career in Australia. He has a diversified background in a wide range of industries with roles in finance, audit, real estate, merchandise trading and travel, as well as logistics.
Mr. James Topham (Proposed Director)
Mr. Topham is an experienced executive with expertise in finance, accounting, auditing and entrepreneurial technology companies. He was an audit partner leading KPMG's Technology Group in the Vancouver office for 20 years where he worked with many fast growing public companies and was involved in many M&A and IPO transactions in Canada, the US and Europe. Mr. Topham founded Social Venture Partners Vancouver in 2001 with a mission to strengthen the organizational capacity of innovative non-profits serving children in-need and youth at-risk. It has funded several million dollars and provided thousands of hours of executive time mentoring these local non-profits. Since retiring at KPMG 7 years ago, Mr. Topham has worked on several Boards of both public and private technology companies. He received a lifetime achievement award from the BC Technology Industry Association and was awarded the designation of Fellow Chartered Public Accountant (FCPA) from the Chartered Public Accountants of BC for his career achievements in the profession and community. He was a founder and Board member for 9 years of the BC Technology Industry Association that represents the technology industry in BC. Mr. Topham is a CPA and has a Bachelor of Commerce degree with Honours from the University of Saskatchewan graduating as the most distinguished graduate in the College of Commerce.
Mr. Allen Ma (Proposed Director)
As a 30-year technology industry veteran, Mr. Ma was the CEO of Hong Kong Science & Technology Parks before he retired in July 2016. He held senior executive positions within the information and communications technology sector. His past roles include president for Asia-Pacific at British Telecom, vice-president for Asia at the global telecom solutions sector of Motorola, executive director of Hong Kong Telecommunications - subsequently called Cable & Wireless HKT - and managing director of Hong Kong Telecom CSL. Ma holds an MBA from the University of Toronto and is a fellow member of both the Chartered Institute of Management Accountants, UK and the Association of Chartered Certified Accountants, UK. He is also a Certified Management Accountant of Canada.
Proposed Advisory Team
Novoheart is supported by a Scientific Advisory Board whose proposed composition consists of eminent scientists renowned in the fields of stem cells, cardiac biology and physiology, tissue engineering, and clinical cardiology including clinical trials research, from top academic research institutes in the U.S.A. Their technical expertise will guide the development of Novoheart as a forerunner in the application of cutting-edge technologies to develop new and better treatments for heart disease and beyond.
Further Details
Both the Company and Novoheart intend to work diligently to complete the conditions precedent to Closing and anticipate completion of the Transaction in the second quarter of 2017. The Company will update its shareholders with further details as they become available.
ON BEHALF OF WOODROSE VENTURES CORPORATION
Darren Devine, President, CEO and Director
NEITHER THE TSX VENTURE EXCHANGE NOR ITS REGULATION SERVICES PROVIDER (AS THAT TERM IS DEFINED IN THE POLICIES OF THE TSX VENTURE EXCHANGE) ACCEPTS RESPONSIBILITY FOR THE ADEQUACY OR ACCURACY OF THIS RELEASE.
Completion of the Transaction is subject to a number of conditions, including but not limited to, Exchange acceptance and if applicable pursuant to Exchange requirements, majority shareholder approval. Where applicable, the Transaction cannot close until the required shareholder approval is obtained. There can be no assurance that the Transaction will be completed as proposed or at all.
Investors are cautioned that, except as disclosed in the Filing Statement to be prepared in connection with the Transaction, any information with respect to the Transaction may not be accurate or complete and should not be relied on. Trading in securities of the Company should be considered highly speculative.
The TSX Venture Exchange has in no way passed upon the merits of the Transaction and has neither approved nor disproved the contents of this news release.
Cautionary Note Regarding Forward-Looking Statements
Information set forth in this news release may involve forward-looking statements under applicable securities laws. Forward-looking statements are statements that relate to future, not past, events. In this context, forward-looking statements often address expected future business and financial performance, and often contain words such as "anticipate", "believe", "plan", "estimate", "expect", and "intend", statements that an action or event "may", "might", "could", "should", or "will" be taken or occur, or other similar expressions. All statements, other than statements of historical fact, included herein including, without limitation; statements about the terms and completion of the Transaction are forward-looking statements. By their nature, forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements, or other future events, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Such factors include, among others, the following risks: failure to satisfy all conditions precedent to the Transaction, including shareholder approval, approval of the TSX Venture Exchange and completion of the necessary financings and the additional risks identified in the management discussion and analysis section of Woodrose Corporation's interim and most recent annual financial statement or other reports and filings with the TSX Venture Exchange and applicable Canadian securities regulators. Forward-looking statements are made based on management's beliefs, estimates and opinions on the date that statements are made and the respective companies undertakes no obligation to update forward-looking statements if these beliefs, estimates and opinions or other circumstances should change, except as required by applicable securities laws. Investors are cautioned against attributing undue certainty to forward-looking statements.
To view the photo associated with this press release, please visit the following link: http://www.marketwire.com/library/20170312-1088577_MyHeart_800.jpg
Originally posted here:
Woodrose Ventures Corporation Announces Proposed Acquisition of Global Stem-Cell Biotechnology Company ... - Marketwired (press release)
